Introduction:
Multiple myeloma (MM) develops over years from the asymptomatic precursors, monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Recent evidence shows that initiating treatment at an asymptomatic stage improves outcomes in MM. However, a vast majority of MM patients are diagnosed at the point of symptomatic malignancy and never have the opportunity to receive early treatment. We aimed to evaluate systematic screening for MGUS as a way to significantly expand the availability of early treatment and improve overall outcomes. Despite the widespread use of cancer screening, the literature on the potential harms of screening, particularly psychological harms, is limited. Studies on the psychological effects of screening are therefore, urgently needed. The Iceland Screens, Treats, or Prevents Multiple Myeloma study (iStopMM) is a population-based screening study and randomized controlled trial (RCT) of follow-up strategies aimed at evaluating the potential benefits and harms of MGUS screening.
Methods:
All residents of Iceland born in 1975 or earlier were invited to participate of whom 80,759 individuals (54% of the eligible population) provided written informed consent for screening. Serum samples collected between 2016-2020 were screened by serum protein electrophoresis (SPEP) and free light-chain (FLC) assay. Those who had MGUS entered a RCT of follow-up strategies. Arm 1 was not notified; Arm 2 was followed according to guidelines; Arm 3 was followed according to a more intensive strategy. Participants who progressed were offered early treatment. Participants were followed to a diagnosis of MM, SMM, Waldenström's macroglobulinemia (WM), smoldering WM, chronic lymphocytic leukemia (CLL), and non-Hodgkin's lymphoma (NHL) with censoring at death. Progression rates were compared using the log-rank test. At registration, all participants were asked to answer the Patient Health Questionnaire 9 (PHQ-9), General Anxiety Disorder 7 (GAD-7), and the Satisfaction with Life Scale (SWLS), validated questionnaires that assess depression, anxiety, and satisfaction with life. These questionnaires were sent annually to all participants and two weeks after MGUS diagnosis to Arms 2 and 3. Scores for Arm 1 and Arms 2 and 3 combined were compared using a generalized estimating equations linear model to account for multiple and differential numbers of observations before and after MGUS diagnosis.
Results:
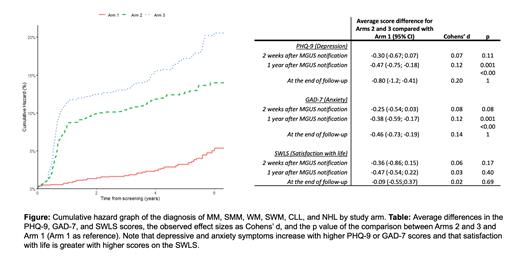
A total of 75,422 participants provided a serum sample for screening and 3,487 had previously unknown MGUS and entered the RCT. Over a median follow-up of 4-4.5 years (depending on the outcome of interest) a total of 33, 136, and 185 were diagnosed with MM, SMM, WM, SWM, CLL, or NHL in Arms 1, 2, and 3 (p<0.001), respectively (Figure). In total, 775 (65%) and 1,838 (72%) participants in Arm 1 and Arms 2 and 3 answered a total of 2,673 and 8,540 questionnaires respectively. PHQ-9 and GAD-7 scores were not higher and SWLS scores were not lower in those who were made aware of their MGUS status (Arms 2 and 3) either at MGUS diagnosis, one year after diagnosis, or at the end of follow-up. Thus, no negative psychological impact was observed. In fact, a small positive psychological impact of MGUS diagnosis was observed as demonstrated by lower scores on PHQ-9 and GAD-7. However, the effect sizes were small (Cohens' d range: 0.02-0.2) indicating no meaningful difference in psychological wellbeing between the groups (Table).
Discussion:
In this first, large population-based screening study for MGUS and subsequent RCT, we found that screening is feasible and leads to early diagnosis and treatment of MM and related disorders. Importantly, MGUS screening was not associated with any demonstrable psychological harm. This shows, for first time, that providing detailed and balanced information at diagnosis, and plans for follow-up can prevent psychological harm from the diagnosis of this precancerous condition, a finding which is of significance to the field of cancer screening in general. These findings are a major milestone towards establishing the role of MGUS screening. If they translate into the prevention of symptomatic malignancies and improvements in overall survival, screening for MGUS can produce a paradigm shift in the field of plasma cell disorders towards a future of early detection, early treatment, and prevention, and perhaps even a cure.
Disclosures
Hultcrantz:Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria; Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding. Harding:Bindingsite ltd.: Current Employment, Membership on an entity's Board of Directors or advisory committees. Landgren:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Adaptive: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Theradex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees. Kristinsson:Celgene: Research Funding; Amgen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal